Checkpoint inhibitors are no longer reserved for late-stage salvage. The clearest signal from recent meetings and trials is this: the greatest survival benefit may be increasingly found in neoadjuvant and perioperative settings.
This shift introduces both opportunity and complexity – demanding faster diagnostics, tighter surgical coordination, and more adaptive post-op pathways.
Here’s a curated summary of significant trends emerging from recent data, tumor by tumor, with the nuances that actually drive decision-making:
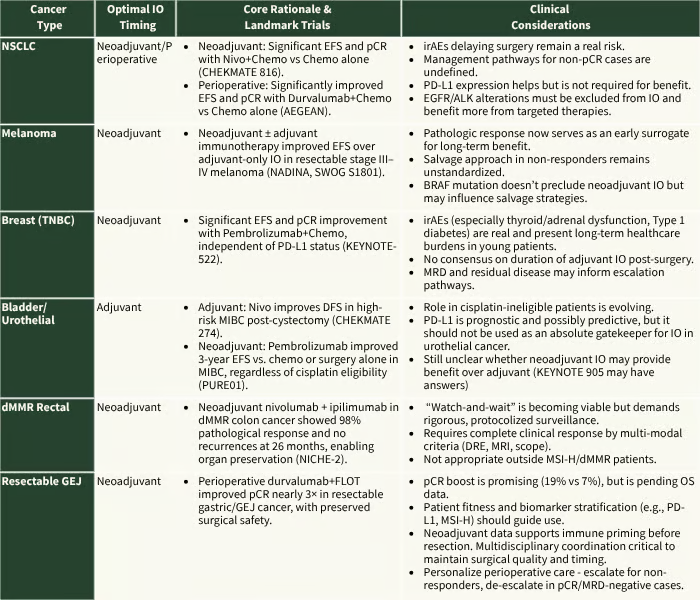
CHECKMATE 816; AEGEAN; NADINA, SWOG S1801, KEYNOTE 522, CHECKMATE 274, PURE 01, KEYNOTE 905, NICHE-2, MATTERHORN
Updated Frameworks Worth Operationalizing
As immunotherapy moves earlier in the treatment pathway, precision in execution—not just decision-making—is the new frontier. Here’s how implementation frameworks can evolve based on the latest cross-cancer learnings.
1. Managing Immune-related AEs (irAEs) That Threaten Surgical Timing
- Pre-agreed surgical timing buffer: Ensure upfront alignment with surgeons before neoadjuvant starts—especially for tumors where a narrow resection window is critical.
- Nurse-led symptom tracking: Use structured checklists at every touchpoint to flag irAEs early; the goal is intervention at Grade 1–2, not 3+.
- Fast-lane EMR orders: Preconfigure steroid/immunosuppression protocols based on ASCO/NCCN-compliant templates to avoid delays.
2. Adjuvant IO Decisions in non-pCR Patients
- Trigger thresholds: ≥50% viable tumor post-neoadjuvant should auto-trigger MTB discussion for escalation.
- Risk-adapted models: Define clear escalation paths—continue IO, add dual checkpoint, or enroll in a trial.
- Leverage ctDNA (where available): While not yet universally reimbursed, post-op MRD testing with ctDNA is emerging as a prognostic tool to guide adjuvant intensification.
3. Non-Operative Management in Select Cancers
- cCR triad: Stick to the holy trinity—negative MRI, biopsy, and DRE—before offering non-operative management.
- Documented consensus: Formal MTB signoff (not solo clinician call) is critical; institutional standards must reflect this.
- Surveillance compact: Written patient agreement for intensified follow-up, flagged EMR alerts for any missed imaging.
4. Chronotherapy: When You Infuse Also Matters
- Evidence update from ASCO 2025 Abstract 8516: Among 2,631 patients across 5 cancers (NSCLC, RCC, melanoma, HNSCC, urothelial), IO before ~1PM linked to 8.3-month longer OS.
- Apply it early: Data suggest effect is strongest in the first 3 months of treatment—critical for neoadjuvant/perioperative settings.
- Feasibility-first approach: Default morning slots for IO-only or IO+TKI regimens, especially in high-volume centers. When every 1% improvement counts, time-of-day is a low-burden, high-yield lever.
What This Means for Clinical Teams Right Now
If you’re still starting IO at recurrence, you’re behind.
- Reflex molecular testing must start at diagnosis to catch narrow neoadjuvant windows.
- Multidisciplinary plans need to begin before surgery, not after.
- Infusion timing, often overlooked, is now a controllable factor in patient survival.
It’s critical to also weigh the non-trivial, often unpredictable, and potentially severe toxicities of immunotherapy—and ensure robust management strategies are in place for eligible patients.
When IO is the right choice, success in oncology’s new era will hinge not just on the decision to treat, but on managing timing and toxicity with precision.
Best,
Shruti Agarwal, PhD
Together4Cancer
—————————————
The Compass is your practical briefing on what’s working in oncology care – strategy, science, and systems. No fluff. Just implementation.
Additional Reads:
1. The rapidly evolving paradigm of neoadjuvant immunotherapy across cancer types